Who qualifies as an inventor on a patent is not a matter of opinion—it is a legal determination governed by USPTO rules. This article explains how inventorship is defined, why it matters, and how patent claims can ultimately determine who is named on an issued patent.

Commercialize your technology
As a researcher, consider what you need to do to protect your new discoveries, novel approaches or unique ideas. Or perhaps you see potential for commercial applications. Tech Transfer helps with those tasks as well as facilitating university/industry opportunities ranging from materials transfer and confidentiality agreements to research collaborations and licensing agreements.
Tech Transfer also fulfills funding agency reporting as required by the Bayh-Dole Act (37 CFR 401), to preserve the potential for commercialization, in accordance with the Intellectual Property Policy.
NEW TECHNOLOGY DISCLOSURE FAQ
Yes. Early engagement with UB can help you better understand UB's processes, identify a path forward and assist you in avoiding certain pitfalls. Please contact the UB Incubator Commercialization Manager assigned to your area.
* Because these meetings can be very educational and informative for both parties, UB encourages you to include your graduate students and post-docs.
The Commercialization Manager (CM) will explain the process to you and ask about your technology and area(s) of research before providing recommendations as to whether and when to submit a New Technology Disclosure (NTD). The CM also may offer suggestions for the types of data, results or information that you will need before the invention is ready for the next step. In addition, the CM may request that you complete certain activities (laboratory and/or web-based research) before submitting an NTD.
* Because these meetings can be very educational and informative for both parties, UB encourages you to include your graduate students and post-docs.
Yes you can. However publication or presentation in any form prior to the filing of a patent application may adversely impact potential foreign patent rights and will start the one-year “clock” within the U.S.
It is best to submit a New Technology Disclosure well before communicating or disclosing your invention to people outside your research group.
Public disclosures include:
- Journal publications
- Poster presentations
- Oral presentations
- Grant awards
A New Technology Disclosure (NTD) is a written description of your technology that is submitted to UB.
No. Although the New Technology Disclosure (NTD) is treated as confidential, it does not provide any protection for your technology. Protection is provided only through filings and agreements that take place subsequent to the submission and evaluation of an NTD by UB.
As an obligation of your employment and as specified in the Patents and Inventions Policy of the State University of New York, all inventions made by faculty members, employees, students, and all others utilizing university facilities shall belong to state university and should be voluntarily disclosed, or shall be disclosed to state university upon request of the university.
Under the Bayh-Dole Act of 1980, you also have an obligation to disclose technologies developed with the use of federal funds. UB handles the reporting of such technologies to the sponsoring agency.
Submission of a New Technology Disclosure provides an internal record of the technology’s development. It also enables UB to begin assessment of the technology for protection needs and commercialization opportunities.
We encourage you to submit a New Technology Disclosure whenever you feel you have discovered or developed something unique and which potentially has commercial value. You should make your disclosure long before you or your students present or publish the technology.
You may submit a description of your technology to UB using the New Technology Disclosure form.
The New Technology Disclosure consists of the following parts:
- Title
- Past and planned disclosures
- Funding sources
- Third party agreements (e.g. MTAs, CDAs)
- Marketing targets and contacts, if known
- A detailed description of the technology
- A list of inventors/developers and the nature of their contribution
You may attach documents to the submission. Documents and information that UB finds useful include:
- Manuscripts
- Publications (journal, thesis, etc.)
- Slide decks
- Posters
- Published patents and applications relevant to the technology
- Statements and evidence that distinguish your technology from the prior art
- Descriptions, references or links to competing research groups and/or technologies
- A description of all data, both success and failure data
- All relevant sketches, designs, drawings, images, pictures, etc.
If you have developed tools that would benefit other researchers and you are interested in making them available, UB recommends that you report them via a New Technology Disclosure.
UB considers research tools to include materials such as antibodies, vectors, plasmids, cell lines, mice and other materials used as “tools” in the research process. In general, patents are not required in order to license and commercialize research tools and/or to generate revenue for your laboratory.
If you have research tools that you believe to be valuable, or wish to provide to others (including research collaborators), UB will work with you to develop the appropriate protection, licensing and distribution strategy.
The following is a non-exhaustive list of technologies that should be submitted:
- Research tools (e.g. antibodies, vectors, plasmids, cell lines, mice and research reagents)
- Biologics (proteins, peptides, antibodies and nucleic acids for diagnosing, treating or preventing disease)
- Chemical compositions
- New uses for making or using biologics or chemical compositions
- Medical devices
- Computer software
- Novel materials
- Instruments
- Machines
- Drug delivery technologies
THE COMMERCIALIZATION LIFECYCLE
Research and development work funded by the federal government, foundations or industry is the basis for discovering and developing novel technologies and software.
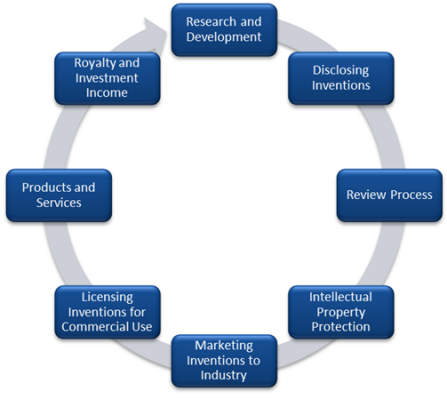
When you believe you have discovered a novel technology or developed a useful new computer software, please submit a description of the technology to Tech Transfer using the New Technology Disclosure form. For additional information about disclosure, please visit our Disclosure page.
IMPORTANT NOTE:
- Submission to Tech Transfer should occur no less than one month prior to any public disclosure.
- Submission to Tech Transfer does NOT confer protection of the technology.
Technology can be:
• Composition
• Device
• Method
• Process
• Biological material
• Research reagent
Public disclosures:
• Journal publications
• Poster presentations
• Oral presentations
• Grant awards
When your disclosure to Tech Transfer is complete, we will make an initial assessment to determine your protection needs and commercialization opportunities. We will work with you during this process.
Time: In most cases, up to three months to complete.
Assessment includes:
• Ownership
• Types of protection
• Market-related factors
Depending on the results of the review process, Tech Transfer will determine whether to:
- File for a patent, copyright or trademark
- Hold the work pending additional development
- Control distribution of the material or reagent in the absence of formal protection
- Close the file
Time: Depending on the type of protection, up to several years (and may cost tens of thousands of dollars)
We achieve greater success in marketing and promoting technologies within the university’s IP portfolio by working together. Many times our most successful leads come from the inventor's industry contacts who serve as internal champions within their own companies and organizations.
Time: 1 month to several years
In cases where multiple companies are interested in the technology, Tech Transfer will identify the most appropriate licensing partner(s). We consider a potential partner’s overall ability to:
- Commercialize the technology
- Obtain or provide funding to support development of the technology
- Benefit the local economy
Other factors include prior and ongoing relationships with the potential partner(s), your willingness and ability to work with the potential partner(s), and the ability of the partner(s) to accept license terms required by RFSUNY and UB.
Though it is always Tech Transfer's preference to work with established companies, there are cases where creation of a new business start-up may be the best or only option for near-term commercialization activities. In such cases, Tech Transfer may assist in planning and creating the start-up.
Licensing
Tech Transfer works on behalf of the university and SUNY RF to negotiate favorable option and license agreements with third parties. Each license agreement is unique, but common elements include licensing fees, patent reimbursements, royalties, and commercialization milestones.
Time: several months to a year or more
Commercialization
UB technologies licensed by third parties often require further research and development prior to the launch of a new product or service. In some cases, you may have the opportunity to participate in this process through research and/or consulting activities.
Time: several months to several years or more depending on the need for regulatory approvals
Revenues generated from the licensing and commercialization of UB technologies are paid back to the inventor(s) and university according to the Royalty Distribution Policy. This income further fuels research and development programs at UB and may lead to future technologies and software.
PATENTS
The U.S. Patent and Trademark Office (USPTO) grants two main types of patents:
- Utility Patents apply to processes, machines, compositions of matter, articles of manufacture and improvements thereof.
- Utility patents last for 20 years from the application filing date.
- Design Patents apply to the ornamental or aesthetic design of an object or product.
- Design patents last for 15 years from the issue date of the patent.
In order to be deemed patentable, an invention must meet certain requirements:
Fall within Patentable Subject Matter
Certain subject matter (e.g. laws of nature, naturally occurring genes and organisms, etc.) are excluded from patentability even if the other requirements are met.
Novel
Is the invention new? This requirement is met provided there is no single reference prior to the date on which the patent application was filed wherein such reference encompasses all elements of the claimed invention. There are slight differences in the U.S. definitions when compared to other countries.
Non-obvious
In light of what is already known and has been disclosed, is the invention obvious to a Person Having Ordinary Skill in the Art (PHOSITA)?
If there are multiple references, when combined, that ecompass all elements of the claimed invention, then it's difficult to meet this requirement.
Useful
Does the invention have a useful purpose or application? While the threshold for meeting this requirement is low, there should be some demonstration or support for the utility of the invention.
In addition to the above, the application must meet the written description and enablement requirements. To meet these requirements, the application must provide a complete description for how to make and use the claimed invention and should provide enough evidence to support the full breadth of what is being claimed. Failure to provide such support can result in a significant narrowing of claim scope, which may have an adverse impact on licensing potential.
Patent examiners will look at the prior art when evaluating an application’s ability to meet the patent requirements. Prior art includes all information that has been made available to the public anywhere in the world, in any form, prior to the date on which the patent application was filed. Prior art includes publications, websites and public demonstrations.
While patents and patent applications often contain very broad descriptions of technology, protection is afforded only to what is claimed. Patent claims define the metes and bounds of the protection. Claims can be found at the end of issued patents and patent applications. In order to be considered infringing, a technology must read on all elements of a third party’s claim.
It is critically important to name the proper inventors on a patent. Failure to do so, either by including someone who is not an inventor or by failing to include someone who is an inventor, could result in the patent being invalidated.
Inventorship is a legal determination and should not be confused with authorship for a journal publication. An inventor is someone who contributed to the conception of at least a portion of at least one claim.
Please note that inventorship can change between the time a patent application is filed and the time it is issued. As claims are canceled or amended during the prosecution process, the list of inventors also may change. Outside counsel will help you determine who should and should not be included as an inventor. Counsel will do so when the patent is filed and when it is issued.
While the patent process in the U.S. is similar to that in other countries, there are some key differences with respect to meeting the patent requirements and for adding new subject matter.
Unlike most other countries, the U.S. offers a one-year grace period for meeting the novelty requirement. That is, if an inventor publicly discloses an invention prior to the date on which a patent application is filed, the claimed invention would still meet the novelty requirement if such disclosure took place no greater than one year prior to the date on which the filing took place. Most other countries follow an absolute novelty requirement: if a disclosure takes place prior to filing, all potential patent rights are lost for the elements that have been disclosed.
The U.S. also allows an applicant to add subject matter to a patent application through a continuation-in-part (CIP) application, which allows the applicant to later claim priority to the filing date of the original patent application. There is no foreign equivalent, so new patent applications must be filed in foreign countries. However, in such cases the applicant’s earlier filing can be used to establish arguments against the patentability of the later-filed application.
Provisional Patent Application
Like most other university technology transfer offices, STOR typically initiates the patent process through the filing of a provisional patent application. A provisional patent application is a relatively inexpensive means for temporarily preserving patent rights for an invention prior to its disclosure.
- Provisional patent applications expire one year from the date on which they are filed;
- Are not reviewed during that period;
- Do not require claims; and
- Are never published.
However, they serve to establish an early filing (aka priority) date for only the material that is adequately described and enabled within it.
Priority dates are important for determining what prior art is relevant when considering the patentability of a claimed invention. Only prior art that was publicly available prior to the priority date may be considered when evaluating the patentability of a claimed invention. Early priority dates are the goal.
Though these applications are not reviewed during their one-year term, it is important that they adequately describe and provide support for any invention claimed within a later-filed application since the patent office will review and scrutinize the provisional patent application during prosecution of the later-filed application claiming priority to it.
- In such cases, if the claimed invention is not fully described and supported within the provisional patent application, different priority dates may be assigned.
If new material is developed following the date on which the provisional application is filed, you should consult STOR prior to any public disclosure of such material.
PCT Application
We typically file a PCT application if:
- The potential remains for securing foreign patent protection;
- The non-U.S. markets are attractive for products and/or services based on the technology; and
- STOR elects to convert the provisional patent application to a full patent application.
This filing, which must take place on or before the expiration of the Provisional Patent Application, makes the process of international filings easier and less expensive in the short term. These applications undergo a cursory review, however, the results of such review, known as the International Search Report and Written Opinion, are not binding on any national or regional patent office.
If prior disclosure or market(s) being limited mainly to the U.S. caused the filing strategy to bypass the PCT application, a U.S. Non-Provisional Patent Application is the typical filing that takes place prior to the expiration of the Provisional Patent Application. When an applicant “nationalizes” a PCT application in the U.S., we must file a U.S. Non-Provisional Patent Application.
- Assuming they claim priority to an earlier filed Provisional Patent Application, these applications are typically published within six months from the date on which they are filed (18 months from the earliest filing date, inclusive of any provisional applications to which it claims priority).
Please note that these applications are expensive with typical preparation and filing costs ranging between $8,000 and $15,000.
National Stage Patent Applications
Beginning approximately 30 months from the date on which the earliest application (typically the provisional patent application) was filed, STOR must decide whether, and in which countries and/or regions (e.g. Europe), to file national stage patent applications. The filing fees are relatively high and we could incur additional costs for any required language translations.
The cost to nationalize a PCT application typically ranges from $2,000 to $10,000 exclusive of prosecution costs and annuities that may be required to keep the application alive.
Patent Prosecution
For each country or region in which an application is nationalized, the applicable patent office reviews it for form and content, primarily to determine whether the claimed invention meets the patent requirements. Typically within a year from the filing date of a national stage patent application, the patent office will notify you as to whether the application and its claims have been accepted.
- Often we refer to this notification and subsequent patent office communications as office actions.
Frequently the patent office will reject the application for failing to meet certain formalities or because the patent office argues that the claims are not patentable over the “prior art”. We use this opportunity to begin negotiations with the patent office.
- You, as the applicant, will need to work with outside counsel in developing counter-arguments to overcome the rejections and maximize the scope of your claim.
- This process may take two or more years.
In some cases, the patent office may argue that multiple inventions are claimed within a single application. You then must select one invention (restriction requirement) so that the patent office can begin its evaluation. This choice does not preclude you from seeking protection for the non-elected inventions. However, you must pursue the non-elected inventions in a separate application, often referred to as a divisional application.
Depending on the nature, complexity and number of rejections issued by a given patent office, each response to an office action may cost between $1,000 and $5,000. It is not uncommon to receive two or more office actions for an application within each country in which it was filed.
Notice of Allowance
If the patent office deems at least one claim to meet the requirements, it will issue a notice of allowance (“NOA”). When you receive an NOA, you must decide whether you want to file any divisional, continuation or continuation-in-part applications. If so, then you need to file those additional applications prior to the issue date of the allowed application. It's also a good time to review the list of inventors against the claims that are about to issue.
Divisional applications are described above.
A continuation application is an application that claims an invention that was: (a) not previously claimed; and (b) fully supported within the originally filed application.
A continuation-in-part (CIP) application is one in which the applicant adds subject matter (data and support) that was not present in the original application. It is a convenient way for you to claim improvements developed after the original application was filed.
Issued Patent
When you have paid the issue fee and met all other requirements (e.g., drawings) within the allotted time, your application will be assigned a patent number and issue date. Once the patent is issued, you, as owner or licensee, may pursue any infringers, even if the infringement happened while the application was pending.
Maintenance Fees
In order to maintain the patent, you must pay periodic maintenance fees or annuities in each country issuing a patent. These fees typically increase over time.
Challenge
Just because a patent issues does not mean that it is valid and enforceable for the duration of its term. People may challenge your patent with resulting outcome(s) that might strengthen, weaken or even eliminate your patent.
Expiration
Utility patents expire 20 years from the filing date of the earliest application to which priority is claimed (excluding provisional applications). A patent office might extend this term if the office failed to meet certain deadlines during prosecution. A terminal disclaimer also might reduce the term.
You can find term adjustments in the Notice Section on the cover page of issued U.S. Patents.
Please note that anyone may practice the invention once a patent expires.
Abandonment
You can abandon the patent process at any time by failing to file an application, respond to an office action or pay a fee.
Please note that STOR reserves the right to abandon an application at any time and for any reason.
OTHER IP PROTECTIONS
A copyright is a right granted by U.S. federal law that allows its owner to exclude others from:
- Reproducing the work
- Preparing derivative works
- Distributing the work via public sale, rental, lease or lending
- Public performance or display of the work
Copyright arises automatically at the moment an original work is fixed in a tangible medium. Registration of a copyright is not required but does enable its owner to pursue infringers.
Duration of Protection: life of the author + 70 years, or 95 or 120 years, depending on the nature of authorship
A trademark (TM) or servicemark (SM) is used to protect a word, symbol or phrase, and in some cases, the color or packaging of a product. Trademarks are primarily used to identify the source or quality of goods or services.
Duration of protection: potentially forever
Trade secrets are protections afforded to certain information without formal registration but which must meet the following conditions:
- The information must be secret;
- It must have commercial value; and
- It must have been subject to reasonable steps by its owner to keep it secret
In the university setting, it's often difficult to develop and maintain trade secrets because of our mission to share knowledge through publication and presentation. However, faculty expertise and knowledge may have value which could be transferred to third parties under a license agreement.
Duration of protection: potentially forever
Protecting technologies such as biological materials (e.g. antibodies, cell lines, animal models, etc.) and research reagents might most reasonably be done by controlling their distribution. Often it is easier for customers to purchase materials and reagents rather than recreate them in their labs. Frequently commercial licenses for such technologies can be granted to companies for sale and distribution to the research community.
Engaging with Industry
Engaging with industry leaders could turn your research into an innovative product or service that benefits local and global communities. UB helps the process by negotiating and executing all Material Transfer Agreements (MTA) and Non-Disclosure Agreements (NDA) in keeping with the university's research guidelines and its obligations to federal research sponsors.
The terms in a Material Transfer Agreement (MTA) define the permitted use of materials for non-commercial research only, for a specific research project, and not for use with humans. MTA terms protect the research interests of the providing scientist, as well as protecting the providing institution from any liability that might arise from the recipient's use of the materials.
Procedure for MTAs
You must have a fully executed MTA before you can send or receive materials to/or from a third party.
If you are the providing scientist, you can initiate the process by completing an outgoing MTA Request form.
- UB will prepare the MTA when it receives your completed form.
- UB may contact you with questions when preparing and negotiating the MTA.
- UB will notify you when it is executed so that you may transfer the requested materials to the recipient.
If the recipient/sender is another nonprofit institution, you can expedite the process by using a Uniform Biological Material Transfer Agreement (UBMTA) implementing letter or a simple letter agreement for the transfer of research materials.
- UB will decide based on your completed request form.
- If the recipient/sender is a commercial entity, the preparation and negotiation of an MTA is more complex and often takes longer to finalize.
- If you are the receiving scientist, you can contact UB by submitting the Incoming MTA Request form.
UB has partnered with Kerafast, a company that commercializes unique consumable laboratory reagents developed in UB labs such as: plasmids, antibodies, cell lines, toxins, peptides, viruses, and enzymes.
Advantages of the the Kerafast Blanket MTA
Kerafast has a blanket MTA with UB and can streamline the marketing and distribution of unique lab reagents. Kerafast will set up a web page for the product, as well as for the investigator. Additionally, Kerafast will advertise the product, send targeted e-mails, and deal with customer service interactions to distribute the reagents worldwide. In return, the PI receives a percentage of the profit as a royalty payment every quarter. The agreement is non-exclusive so that one can still share reagents freely with other investigators.
If you are interested in more details, please contact the UB Kerafast representative:
Confidential Disclosure Agreements (CDA) preserve intellectual property protections, allowing the exchange of secret or proprietary information in negotiating future sponsored research agreements, clinical trial agreements, research collaborations or license agreements.
When we receive your initial request, we will assign your CDA for review and, if necessary, begin negotiation. We may contact you with follow-up questions.
If you are discussing collaborations or sponsored research with a company, please first contact Sponsored Project Services (SPS) to begin developing the scope of work, budget and contract.
Most federal agencies are required to allocate a portion of their funding to Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) grants. Both programs require a company to be the applicant. This provides an opportunity for researchers to either collaborate with an established company or consider starting a company.
We work with SUNY Research Foundation (RF) to manage research and grants through Click. Learn More
Ready to connect with UB's innovation ecosystem?
UB faculty innovations and research go beyond campus labs to have real-world impact. Our team matches Western New York companies, startups, and organizations with beneficial resources at UB to help businesses, and the local economy, thrive.
Policies and Guidelines
RESEARCH FAQ
Yes, but because potential patent rights are impacted by these activities, it is best to submit a New Technology Disclosure well before communicating or disclosing your invention to people outside your research group.
Publication or presentation in any form prior to the filing of a patent application may adversely impact potential foreign patent rights and will start the one-year “clock” within the U.S.
Public disclosures include:
- Journal publications
- Poster presentations
- Oral presentations
- Grant awards
Yes, but Technology Transfer will need to know what, if any, third party contracts (e.g. confidentiality, sponsored research and material transfer agreements) may exist since those agreements often govern the conditions of use and influence ownership and licensing rights of your research results. These agreements also typically include notification and reporting requirements.
Yes, but Technology Transfer should be notified in advance of such sharing.
- If you wish to send materials to third parties, please complete an outgoing Material Transfer Agreement.
- If you wish to share other intellectual property with third parties, you might need to complete a Confidential Disclosure Agreement (CDA) / Non-Disclosure Agreement (NDA) to protect your research results and/or intellectual property.
- If you wish to collaborate with a third party, Technology Transfer and/or Sponsored Project Services will assist you in selecting and negotiating the most appropriate agreement(s).
Yes. In all its licensing agreements with third parties, Technology Transfer uses best practices to ensure that it retains the right to practice inventions and to use associated information and data for research and educational purposes, including research sponsored by commercial entities.
Yes; provided that your new employment is with another academic research institution. In such cases, Technology Transfer will work with your new institution to execute an Academic Use Agreement that will enable you to practice the invention for research and educational purposes.
Consulting activities are considered to be outside the scope of your employment. As such, these agreements are not handled or reviewed by the university or by Technology Transfer. However, you should familiarize yourself with the policies of your school, college or department relevant to consulting activities. You also are expected to ensure that the terms of the consulting arrangement are consistent with university policies, including those related to IP ownership, employment responsibilities and use of intellectual property.
The Sponsored Research Agreement (SRA) will specify the Intellectual Property (IP) rights of the sponsor. The university generally retains ownership of patent rights and other IP resulting from sponsored research. However, the sponsor may have rights to obtain a license to the IP resulting from the research. The sponsor generally will not have contractual rights to discoveries that are clearly outside of the scope of the research or that were invented prior to the research contract ("Background IP"). Therefore, it is important to define the scope of work within a research agreement and to review the IP provisions in the research contract.
INSIGHTS FOR RESEARCHERS
Our team publishes articles regularly to keep researchers up to date and answer frequently asked questions (FAQs) about the technology transfer process.
As you may be aware, UB faculty and staff are obligated to assign certain intellectual property under the SUNY Patents and Inventions Policy. A new technology disclosure (NTD) is the first step in fulfilling that obligation. An NTD is simply the submission of a summary of the intellectual property to the Technology Transfer Office (TTO). Intellectual property includes potentially patentable inventions, tangible research materials, computer software, and any unique or novel innovation in the technical arts or any new and useful improvements thereof.
Sponsored research and testing are different types of projects that require different agreements. Generally, research projects that are funded by a company or organization are referred to as sponsored research; and testing is a service that is performed to collect and send data to the company for their evaluation and analysis. This article provides the distinguishing features between these types of agreements and guidance on how to submit a request to our team.
A Material Transfer Agreement (MTA) is a contract between a provider of a material and the recipient that memorializes the rights and responsibilities regarding research material. It defines who owns the material and provides the associated rights and responsibilities regarding that material.
An NDA is a Non-Disclosure Agreement, sometimes referred to as a Confidential Disclosure Agreement (CDA). This type of agreement provides terms that will protect against the unauthorized use or disclosure of any shared confidential information. Below is a summary of important points to know when requesting an NDA.








